Honeycomb Monolith
Honeycomb Monolith, Reducingthecost of VOC control in the semiconductor industry.
Theregenerativethermaloxidizer(RTO)is one of the standard pieces of equipment used to control theemissionofvolatileorganic compounds(VOCs) inthesemiconductorindustry.In normal operation, anRTOremovesVOCsusinggas-phase free-radicalreactionsofhomogeneousoxidationtoCO2 andwaterat1450ºFto1600ºF.Honeycomb Monolith
An RTO uses a regenerative heat exchangeintwoormorepackedbedsoperated with periodic flow reversals. The beds,filledwithaninert ceramic media, areconnectedbyacombustionchamberwhereone or more fuel burnersareinstalledforsystemstartup, and to maintain necessarytemperatureatlowconcentrationsof VOCs.The TheVOC-laden air enters theoxidizeratlowtemperature andisheatedthrough the heat exchangerwiththeinletceramic beds. This airstream then reacts inthecombustionchamberand returnsheattothe outlet beds, where it is absorbed for the next cycle. Uponflowreversal, the bedfunctionschange suchthatasubstantialfractionof energy from VOCcombustionandburnerfiringisregenerated in the upper fraction of the beds.Amplesurfaceareaofceramicmaterial results in highthermalefficiencyachievingupto95percent in well-designed systems.
Despite the high degree of energy regeneration, RTOscanstillrequirehigh fuel consumption –especiallyathighairflowrates. This is particularly trueinthesemiconductorindustry, where large volumes of air at low-VOC concentrations are the norm. An alternative tothermaloxidationis a catalytic processthatoccursat lower temperatures –600°F to900°F. As a resultoftransitionto aregenerativecatalyticoxidizer(RCO), fuel consumption can be dramatically reduced, and inmanysituationstheinvestment in a catalyst is returned in Avery short period of time due to the fuel savings.
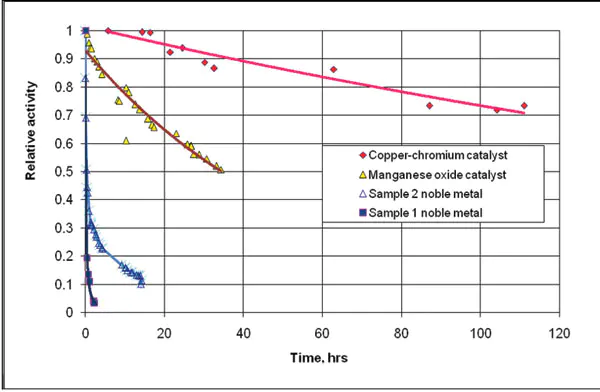
Figure 1: Catalyst testing results. Test conditions: catalyst temperature 750 ºF, 2500 ppm of propane, and 50 ppm of Si(CH3)4 mixed with air in inlet gas.
Semiconductor case study

Figures 2a and 2b show the top layers of ceramic media before catalyst loading in 2005. Figure 2a: Top bed in one of the canisters
Honeycomb Monolith
The conversion of an RTO at a large semiconductor facility inTexas demonstrates that some VOC control challenges for that industry can be overcome.
A key element of the technology wasasilicon-resistant catalyst, which was able to withstand poisoningbysilicon-organic compounds present in the exhaust. Also, prior tothecatalystloading, the facility implemented a series ofmodificationsinexhaust enclosure and distribution to remove the silicon-containing-compounds integral to processingsemiconductorsfrom the treated stream. The catalytic oxidizer had been operated for more than four years at900°F to 950°F in a combustion chamber, compared to the original operating temperature1,500°F. Temperaturereductionresulted insubstantial fuel savings. The exhaustenclosuremodification, combined with the RTO conversion to an RCO, also avoided bed plugging by silicon that occurred there before the conversion. Silicon-resistant catalyst.
While the addition of catalysts to RTOs has beenanacceptedpracticefor several years, it has not been a viableoptionforthesemiconductor industry. Exhaustfromsemiconductormanufacturingoperations contain silicon-organic compounds such asthehexamethyldisilazane (HMDS)commonly used in fabrication as an adhesion promoter on the wafer surface. In atypical RTO, the MDS would oxidize in the combustion chamber andformSiO2compounds. These so-called “sand” particles would build up over time in the unit, and result in pluggingtheceramicmedia, channeling the airflow, and increasingpressuredropacross the beds (see Figure 2).

Figure 2b: Single monolith plugged from its top.
Honeycomb Monolith
Inan RCO, when a volatile molecule containing silicon atom(s)reacts with the catalyst surface, a practically unbreakable bond is created between the active surface site and the silicon atom, inhibiting any catalytic activity of that site. Deactivation by silicon is referred to as masking. It is especially harmful to common platinum-metalVOC oxidation catalysts, containing relatively few, albeit very active catalytic sites. Another type, the so-called “transition” or”base-metal” catalyst, contains a few orders of magnitude greater number of active sites, and thus presents a good opportunity for treatment of gases laden with silicon-containing VOCs.
Several base-metal catalysts were synthesized andtestedinsimulatedreactions of VOC oxidation under theinfluenceofsilicon-containing organics. Figure 1showstimedependencies for catalyst activity during propane oxidationinthepresence of 50 ppm of tetramethylsilane. The testswereperformedin a laboratory reactor with intense internal gasmixingthatyielded the reaction rate data. Relative activity inthechartordinate was calculated as a ratio between runningandinitialrates of oxidation. Two noble metal catalyst samplesweretestedalong with the base metal catalysts.
Sample1inFigure 1 represents a common wash-coated noblemetalcatalystwithactive metals distributed over a thin filmofporousaluminadeposited on a non-porous ceramic carrier. Anothernoblemetalcatalyst, Sample 2 in Figure 1, was obtainedbyimpregnationamassive a highly porous alumina carrier withnoblemetalsolutions. The base metal catalysts tested in Figure1includedmanganeseoxide and copper-chromite catalysts,bothobtainedthroughextrusion of mixes of aluminum hydroxide andbasemetaloxides, followed by subsequent drying and thermal treatment.
Although the impregnated noble metal catalyst(Sample2)demonstrate higher stability than wash-coated (Sample1), both noble metal catalysts deactivate very quickly compared tothebasemetalcatalysts. The copper chromium catalyst showedthelowestrate of deactivation among all tested samples.
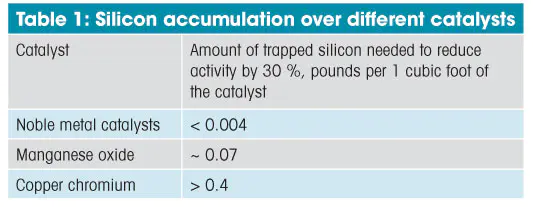
Aside from the VOC oxidation rate measurements,thetestsincludedcontinuous measurements of inlet andoutletconcentrationsoftetramethylsilane, thus it was possibletocalculatetheaccumulation of silicon over the catalyst.Table1presentsthe silicon accumulations over differentcatalystsamples, at whichthe reaction rate of VOC oxidation, was decreased by30percentcompared to the initial rate. This decreasewasnotconsidered high because the rate of reaction couldbeincreasedagain to the initial level through amoderatetemperatureincrease.
The base-metal catalysts can trap a considerablyhigheramountofsilicon than the noble metals (see the comparison inTable1).The most resilient copper-chromium catalyst canabsorb0.4lbs/ft3without a substantial decrease in inactivity. The theexperimentaldatasimilar to that shown in Figure 1 was usedforpredictingcatalystperformance based on informationaboutconcentrationofsilicon-containing organics in actualexhauststream.
RTOretrofit design and installation
Intheinitialstage of the project it was understood thatthecatalyticoperationwould prevent bed plugging due toloweroperatingtemperature. Also, the facility made concerted efforts to remove HMDSfrom the exhaust stream in order to minimizesilicaformation in the oxidizer. This was an additional incentive for theRTO conversion. The catalyst lifetime was estimated at four to five years, based on process gas properties and catalyst testing.
The copper-chromium catalyst recommended for RTO loading–suppliedbyMatros Technologies Inc., Chesterfield, Mo., was produced by extrusion and shaped as Raschig rings (seeFigure3) having both diameter and length of 15 mm. This shapewascompatible with the monolithpacking at linear velocities applied in the RTO. It was determined that adding the catalyst would not increase the bed pressure drop, but rather decrease it because of reduced actual volume of air through the bed at a lower operating temperature. Pressure drop reduction contributed to operating cost savings aside from the reduction in fuel consumption.
Prior to the catalyst installation, the plugged upperlayerofceramicmonoliths was removed, and the remaining bedwascleanedfrom above in every RTO canister. A bulk ceramic mediawasplacedover the remaining3-footbed depth of the monolith. Thecatalystbed, at a depth of 8inches, was placed above the additional bulk media. In addition, a thin layer (3 to 4 inches)of the ceramic media was loaded above the catalyst to protect it from radiant heat emitted by the burner.
It took two days to load the ceramic media,thecatalystandprotective ceramics, and reseal theoxidizerchambers. An additional thermocouple was installed in one ofthecatalystbeds. The control system modification includedreducingtheset-point temperature in the combustion chamber from1,500°Fto950°F, and setting the maximum allowable operatingtemperatureto1,200°F; at temperatures above 1,200°F the copperchromiumcatalystwould begin to break down, and the catalytic action would cease. Theoxidizerwas heated up and put into operation two days after the catalyst loading.
The original burners were designed for hightemperaturesandneededadjustments to operate at lower temperatures.

honeycomb monolith
Retrofitted unit performance

Figure 3: CatalystappliedforRTOretrofit
Ageneraloperatingstrategy for oxidizer control can involvegradualorstepwisetemperature increase with increase insiliconaccumulationover the catalyst. The higher temperature improves the catalyst activity, thus reducing the effect of silicone poisoning. Another strategy is to maintain a fixed operating temperature during most of the catalyst lifetime. This temperature is such that the system will have a sufficient reserve in the activity for achieving high destruction efficiency while the catalyst gradually deactivates. The catalyst activity is periodically monitored (at least annually) usingcatalystsampletesting and field emission tests. The test results determine if the temperature should be increased to compensate forcontinuoussilicondeactivation. Regular annual testing allows the operations team to project the time that the catalyst should be replaced. Once the catalyst begins to lose its effectiveness, the operating temperatures will need to be increased to improvethereactionrates, and fuel costs will increase. To avoid too-highfuelexpenditures, or prevent possible loss of bedmechanicalstrengthdue to high operating temperature, the catalystshouldbereplaced.
Initial performance testing of the retrofittedunitdemonstratedthedestruction removal efficiency of VOCs at morethan99 percent, at a pressure drop slightly lower than in the original to. The concentration of methane was subtracted fromtheconcentrationoftotal hydrocarbons during the testing.
The retrofitted RTO has operated for about four yearswithnochangein the temperature setpoint and pressure drop. Performance monitoring included the oxidizer emissionandcatalystactivitytesting. Most recent field test confirmedthesystemperformance atmore than 97-percent destruction efficiency. The catalyst activity tests showed a moderate activity decrease in line with the expected silicon accumulation and poisoning.
The actual fuel consumption in the original andretrofittedunitswasestimated based on the measured temperatures, VOC loadingsandflowrates. The method of estimations was based onaheatbalanceaccounting for energy spent on heating theprocessgasandcombustion air, and useful heat from VOC oxidation. Theamountofthe combustion air and fuel were assumed to beequaltothedifference between outlet and inlet gas flowrates. Theestimationshowed that the retrofitted RTO decreasedtotalfuelconsumption by two thirds, or as much as 15,000 MCF a year. The system also provided a noticeable reduction in materialandlaborcost for frequent ceramic bed replacement and disposal.
Installation of the catalyst made it possible tocombinethelowtemperature of catalytic oxidation with thehighthermalefficiencyof regenerative heat exchange. These changehadthreeprimaryenvironmental benefits:
1. Due to the much lower oxidation temperature, 700°Fto900°F,theRCO operates using 50- to 60-percent less fuel,andgenerates40-percent less NOX.
2. Due to the nature of the catalyst, a packing moreresilienttoHMDSresulted, thus maintaining high destructionefficiencymuchlonger, improving energy recuperation,andreducingCO2emissions.
3. A reduced volume of packing material for disposal.HoneycombMonolith
Best results and longer operating periods wereobtainedwiththereduction of silicon-containing compounds in the VOC stream.The lesson learned: every effort should be madetominimizeoreliminate HMDS, and to maintain the thehighestoperatingefficienciesin any thermal oxidation system. PE
John D. Miller
j-miller4@ti.com
JohnD.Miller is project manager, Texas Instruments Inc., Dallas. He canbereachedby e-mail at j-miller4@ti.com or call(214)882-4166.
TinaGilliland
t-gilliland@ti.com
TinaGillilandisair-permitting manager at TexasInstruments;e-mailt-gilliland@ti.comor call (972)927-3022.
Grigori.Bunimovich
grigorii@matrostech.com
Grigori.Bunimovichis director of catalyst applications, MatrosTechnologiesInc.,Chesterfield, Mo.; e-mailgrigorii@matrostech.comor call(314)439-9921.
YuriiSh.Matros
yurii@matrostech.com
Yurii Sh. Matros is president of Matros TechnologiesInc.;e-mail:yurii@matrostech.com orcall(314)439-9699.



